Wegovy Weight Loss Pill Shows Remarkable 16.6% Effectiveness in Groundbreaking Clinical Trial
Wegovy Weight Loss Pill Shows Remarkable 16.6% Effectiveness in Groundbreaking Clinical Trial
Table of Contents:
- Breakthrough Trial Results
- OASIS 4 Clinical Trial Details
- How Effective is the Wegovy Pill?
- Pill vs. Injection Comparison
- Benefits for Patients and Healthcare
- Safety Profile and Side Effects
- FDA Approval Timeline and Availability
- Competitive Landscape Analysis
- Additional Health Benefits
- Future of Oral Weight Loss Medications
- Frequently Asked Questions

Breakthrough Trial Results: Wegovy Pill Delivers Impressive Weight Loss
The pharmaceutical world is buzzing with excitement following the publication of groundbreaking results from Novo Nordisk's OASIS 4 clinical trial, which demonstrated that their oral semaglutide pill formulation achieved an remarkable average weight loss of 16.6% over 64 weeks. This represents a significant milestone in obesity treatment, potentially revolutionizing how millions of Americans approach weight management by offering an effective alternative to injectable medications.
The results, published in The New England Journal of Medicine, show that the once-daily Wegovy pill performed comparably to the highly successful injectable version, with over one-third of participants (34.4%) achieving weight loss of 20% or more. This level of efficacy rivals some of the most effective weight loss interventions currently available and marks a pivotal moment in the development of oral GLP-1 therapies.
OASIS 4 Clinical Trial: Comprehensive Research Methodology
:max_bytes(150000):strip_icc()/clinicaltrial-GettyImages-1374014442-7ddfd7ceff6a465ca54fed65d8bd98f3.jpg)
The OASIS 4 phase 3 trial was a meticulously designed 64-week study that evaluated the efficacy and safety of oral semaglutide 25 mg in 307 adults with obesity or overweight conditions accompanied by at least one weight-related comorbidity. Importantly, participants did not have diabetes, making the results particularly relevant for the broader population struggling with weight management.
The study employed a randomized, placebo-controlled design where participants received either the active oral semaglutide treatment or a placebo, alongside lifestyle modifications including dietary counseling and increased physical activity. This comprehensive approach reflects real-world treatment scenarios where medication is combined with lifestyle interventions for optimal results.
Key Trial Demographics and Methodology
The trial population was carefully selected to represent individuals who would most benefit from obesity treatment. Participants had a baseline body mass index (BMI) indicating obesity or overweight status, with additional health conditions such as high blood pressure, high cholesterol, or sleep apnea. The 64-week duration allowed researchers to assess both short-term and sustained weight loss effects, providing valuable insights into the medication's long-term effectiveness.
How Effective is the Wegovy Pill? Analyzing the 16.6% Weight Loss
The 16.6% average weight loss achieved by patients who adhered to the oral semaglutide treatment represents a substantial clinical benefit. To put this in perspective, for a 200-pound individual, this would translate to approximately 33 pounds of weight loss over the 64-week study period. This level of weight reduction can lead to significant improvements in obesity-related health conditions and overall quality of life.

Even more impressive is the fact that over one-third of participants achieved weight loss of 20% or more, compared to just 2.9% in the placebo group. This demonstrates that the oral formulation can deliver clinically meaningful results for a substantial portion of patients, not just modest improvements for the average participant.
Real-World Effectiveness Considerations
When accounting for real-world factors such as medication adherence challenges, the study showed that participants still achieved an average weight loss of 13.6%, which remains clinically significant. This "intention-to-treat" analysis provides a more realistic expectation for patients and healthcare providers, acknowledging that perfect medication adherence can be challenging in practice.
Wegovy Pill vs. Injection: Comparative Analysis
One of the most significant findings from the OASIS 4 trial is that the oral formulation achieved weight loss results comparable to the injectable Wegovy, which has been considered the gold standard for GLP-1 obesity treatment. Previous studies of injectable Wegovy demonstrated average weight loss of approximately 15% over similar timeframes, making the 16.6% result for the pill formulation particularly noteworthy.

The comparable efficacy of oral and injectable formulations is remarkable from a pharmaceutical development perspective. Typically, oral versions of peptide drugs like semaglutide face significant bioavailability challenges, often resulting in reduced effectiveness compared to injectable alternatives. Novo Nordisk's achievement in maintaining therapeutic efficacy in pill form represents a significant technological advancement.
Patient Preference and Convenience Factors
The availability of an oral option addresses a major barrier to treatment adoption. Many patients have needle phobia or find weekly injections inconvenient, leading to poor treatment adherence or complete avoidance of effective therapies. An oral daily pill can significantly improve patient acceptance and long-term treatment compliance, potentially expanding access to effective obesity treatment.
Benefits for Patients and Healthcare Systems
The introduction of an oral Wegovy formulation offers multiple advantages beyond just convenience. For healthcare systems, oral medications typically require less specialized storage and handling compared to injectable biologics. Patients benefit from the discretion and ease of taking a daily pill, which can be seamlessly integrated into existing medication routines.

Moreover, the study demonstrated improvements beyond weight loss, including enhanced cardiovascular risk factors and increased ability to perform daily physical activities. Participants reported improvements in mobility, energy levels, and overall quality of life measures, highlighting the comprehensive benefits of effective weight management.
Accessibility and Healthcare Equity
Oral formulations may improve healthcare equity by making treatment more accessible to populations who face barriers to injectable medications. This includes individuals in rural areas with limited healthcare infrastructure, those with mobility limitations, or patients who require assistance with medication administration.
Safety Profile and Side Effects Analysis
The safety profile of oral semaglutide 25 mg was consistent with the known effects of injectable Wegovy, with gastrointestinal side effects being the most commonly reported adverse events. These included nausea, vomiting, diarrhea, and constipation, which are typical for GLP-1 receptor agonists and generally decrease over time as patients develop tolerance.
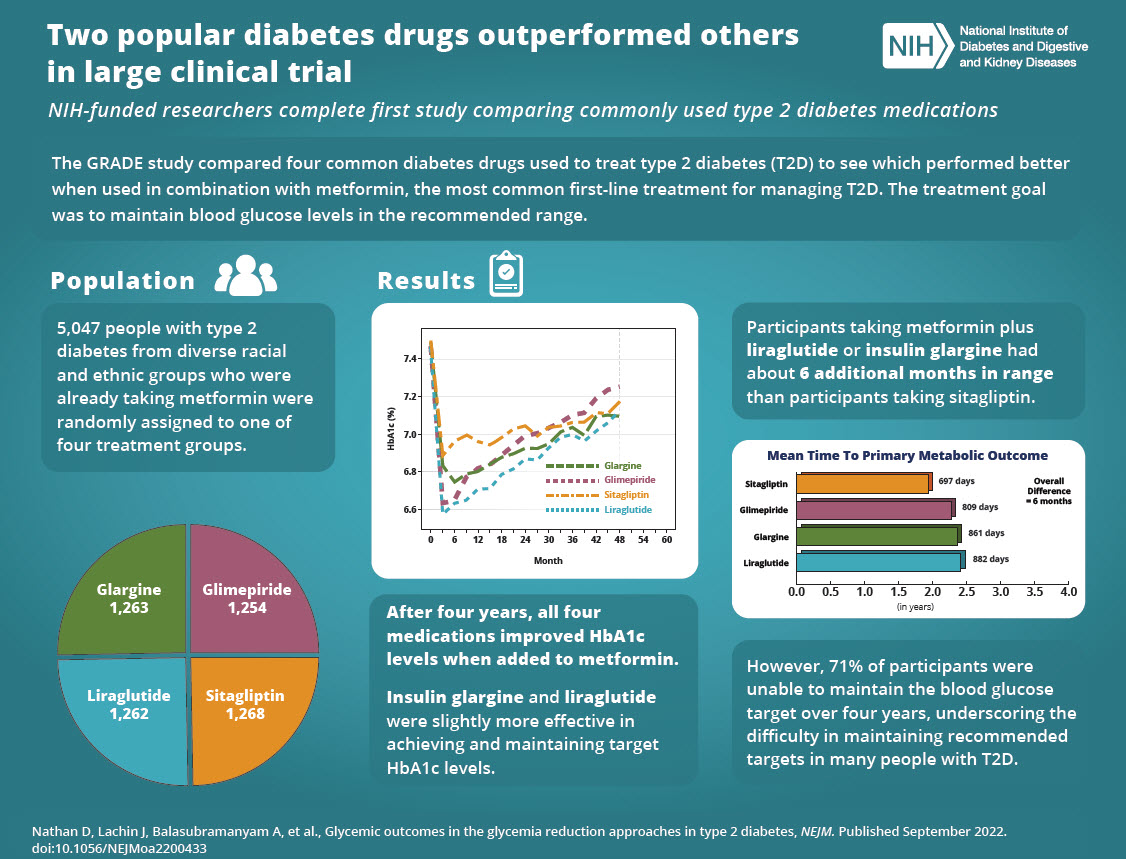
Importantly, the study found no new safety signals or unexpected adverse events with the oral formulation. The discontinuation rate due to adverse events was within expected ranges for this class of medications, suggesting that the oral formulation maintains the favorable risk-benefit profile established by injectable semaglutide.
Long-term Safety Considerations
While the 64-week trial provides valuable safety data, ongoing post-marketing surveillance and longer-term studies will continue to monitor the safety profile of oral semaglutide. The extensive safety database from injectable semaglutide provides reassurance about the long-term use of this medication class.
FDA Approval Timeline and Market Availability
Novo Nordisk submitted the New Drug Application for oral semaglutide to the FDA in February 2025, with a decision expected in the fourth quarter of 2025. This timeline positions the company to potentially be first to market with an oral GLP-1 obesity medication, providing a significant competitive advantage.
The company has already begun manufacturing the oral formulation at U.S. facilities, ensuring adequate supply to meet anticipated demand upon approval. This proactive approach addresses previous supply challenges that have affected access to GLP-1 medications and demonstrates Novo Nordisk's commitment to market readiness.
Manufacturing and Supply Chain Preparation
Novo Nordisk's emphasis on domestic U.S. manufacturing aligns with current healthcare policy priorities and may facilitate faster regulatory approval. The company's investment in U.S. production capabilities also ensures supply chain resilience and may help address pricing concerns related to imported medications.
Competitive Landscape: Wegovy vs. Orforglipron
The race for oral obesity medications intensified this week with Eli Lilly releasing data on their competing oral GLP-1, orforglipron. Lilly's medication achieved average weight loss of 12.4% at the highest dose after 72 weeks, which, while significant, falls short of the 16.6% achieved by oral semaglutide in the OASIS 4 trial.
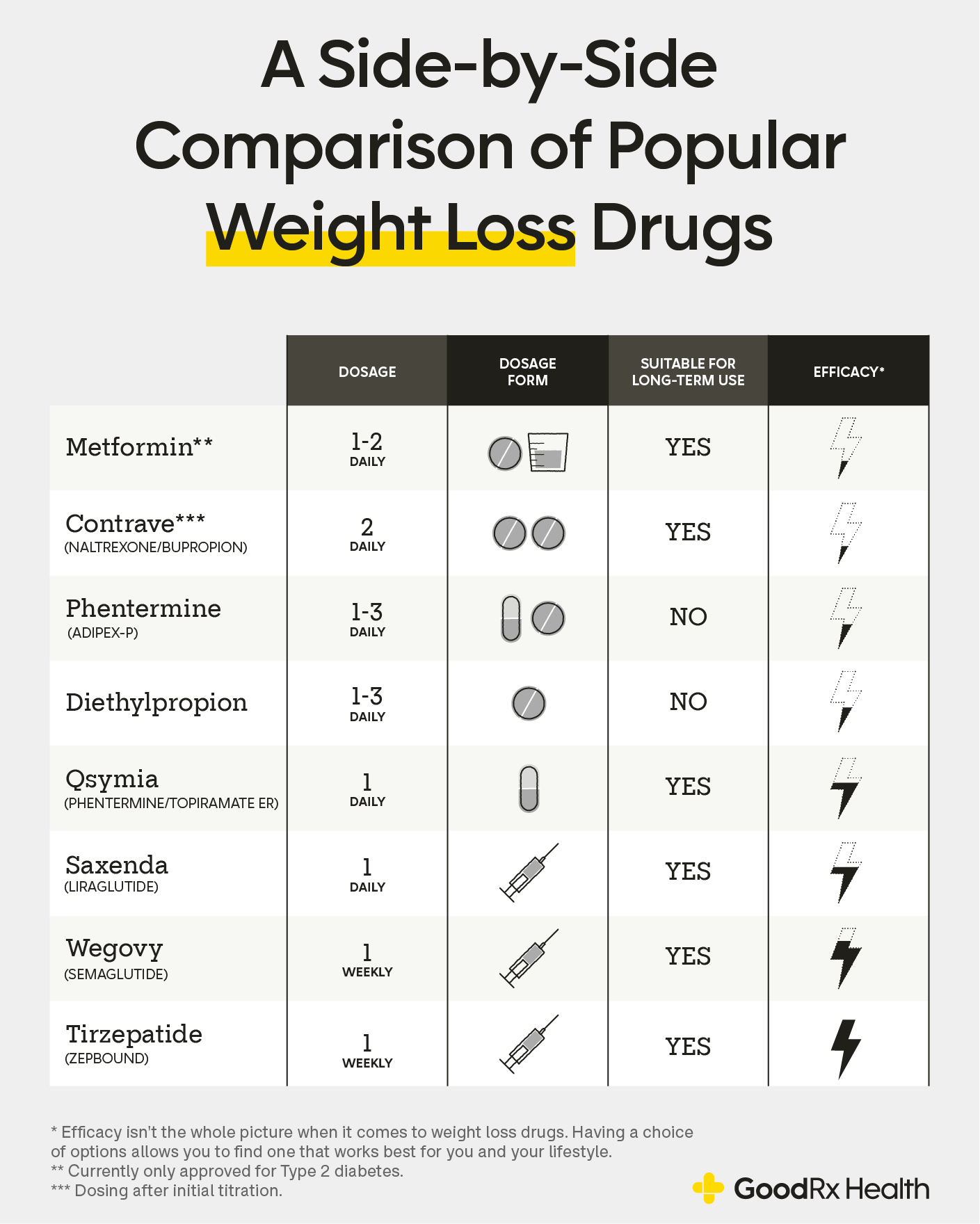
However, direct comparison between trials requires caution due to differences in study populations, methodologies, and outcome measures. Both medications represent significant advances in obesity treatment, and the market may ultimately support multiple oral options to meet diverse patient needs and preferences.
Market Differentiation Strategies
Novo Nordisk's strategy appears focused on superior efficacy and established safety profile, while Lilly may emphasize different aspects such as dosing convenience or specific patient populations. The competition between these pharmaceutical giants is likely to drive continued innovation and potentially improve patient access through competitive pricing.
Additional Health Benefits Beyond Weight Loss
The OASIS 4 trial demonstrated that oral semaglutide provided benefits extending beyond weight reduction. Participants experienced improvements in cardiovascular risk factors, including blood pressure reduction and improved lipid profiles. These effects are particularly important given the strong association between obesity and cardiovascular disease.

Additionally, participants reported enhanced physical function and quality of life measures. The ability to perform daily activities such as walking, climbing stairs, and bending improved significantly, reflecting the practical impact of sustained weight loss on everyday life. These functional improvements often translate to increased independence and reduced healthcare utilization.
Metabolic Health Improvements
Beyond cardiovascular benefits, oral semaglutide treatment was associated with improvements in various metabolic parameters. These included better insulin sensitivity, reduced inflammation markers, and improved sleep quality scores among participants, suggesting comprehensive metabolic health benefits.
Future of Oral Weight Loss Medications
The success of oral semaglutide represents a paradigm shift in obesity treatment, potentially making effective weight loss medications accessible to millions more patients. This breakthrough may accelerate development of other oral formulations and next-generation obesity treatments, creating a more robust therapeutic landscape.
Novo Nordisk is already investigating next-generation compounds, including combination therapies that may offer even greater efficacy. The company's cagrilintide program, which combines GLP-1 activity with amylin receptor agonism, represents the next frontier in obesity pharmacotherapy and could potentially deliver weight loss exceeding 20% in future trials.
Healthcare System Integration
The availability of effective oral obesity medications may reshape how healthcare systems approach weight management, potentially integrating obesity treatment more seamlessly into primary care settings. This could lead to earlier intervention and better long-term health outcomes for patients with obesity-related conditions.
Frequently Asked Questions
How does the Wegovy pill compare to the injection in terms of effectiveness?
The oral Wegovy pill achieved 16.6% average weight loss compared to the injection's approximately 15% in previous trials. Both formulations are considered highly effective, with the pill showing comparable or slightly superior results while offering the convenience of oral administration.
When will the Wegovy pill be available for patients?
The FDA is expected to make a decision on oral semaglutide approval in the fourth quarter of 2025. Novo Nordisk has already begun U.S. manufacturing to ensure adequate supply upon approval, potentially making it available to patients by early 2026.
What are the main side effects of the oral Wegovy pill?
The side effects are similar to injectable Wegovy and include gastrointestinal symptoms such as nausea, vomiting, diarrhea, and constipation. These effects typically decrease over time and are generally manageable with proper medical guidance and gradual dose escalation.
How much weight loss can patients realistically expect?
In the clinical trial, patients achieved an average of 16.6% weight loss when fully adherent to treatment, or 13.6% in real-world conditions accounting for adherence challenges. Over one-third of participants lost 20% or more of their body weight, though individual results vary based on multiple factors.
Will insurance cover the oral Wegovy pill?
Insurance coverage will depend on individual plans and FDA approval status. Given the comparable efficacy to injectable Wegovy and potential cost savings from oral administration, many insurers may provide coverage, but patients should check with their specific insurance providers for coverage determination.
Can the oral pill be used by people with diabetes?
The OASIS 4 trial specifically studied participants without diabetes. However, semaglutide is already approved for diabetes treatment, and ongoing studies are evaluating the oral formulation in diabetic populations. Patients with diabetes should consult their healthcare providers about appropriate treatment options.
Conclusion: A New Era in Obesity Treatment
The remarkable 16.6% weight loss achieved by oral semaglutide in the OASIS 4 trial represents a watershed moment in obesity treatment. This breakthrough offers hope to millions of Americans struggling with weight management by providing an effective, convenient alternative to injectable medications. The combination of proven efficacy, manageable side effects, and oral convenience positions this treatment to significantly expand access to effective obesity therapy.
As we await FDA approval in late 2025, healthcare providers and patients can look forward to having a powerful new tool in the fight against obesity. The success of oral semaglutide also validates the potential for continued innovation in this space, promising even more effective treatments in the years to come.
Stay Informed About Weight Loss Breakthrough
Don't miss updates on the latest developments in obesity treatment and the Wegovy pill approval process. Consult with your healthcare provider to determine if emerging weight loss medications are right for your health goals.
Take control of your health journey with the latest advances in weight management science.
